Homologous concentrating on nanoparticles for enhanced PDT in opposition to osteosarcoma HOS cells and the associated molecular mechanisms | Journal of Nanobiotechnology
Preparation and characterization of MH-PLGA-IR780 NPs
MH-PLGA-IR780 NPs, a homologous concentrating on NPs with a shell/core construction (MH outdoors the shell and IR780 contained in the shell) had been ready utilizing a double-emulsion method and the bodily extrusion methodology. The engineered NPs achieved tumor concentrating on to the HOS cell line and had superior FL/PA imaging efficiency in vivo. Underneath NIR irradiation (808 nm), focused PDT had the potential to trigger mitochondrial dysfunction and led to the extreme accumulation of ROS, LPOs, and Lipid-ROS. Apoptosis and ferroptosis acted as imply demise modes of the constructed concentrating on NPs-mediated PDT. The precise molecular pathway of apoptosis was revealed by the discharge of cytochrome c-activated mitochondrial apoptosis, and the activation of NCOA4-mediated ferritinophagy and the passivation of GPX4 synergistically induced one other RCD, ferroptosis. Extra importantly, the connection between cell apoptosis and ferroptosis induced by this focused theranostic nanoplatform-mediated PDT was verified by the pretreatment with ferroptosis inhibitors (DFO and Fer-1), and it was lastly demonstrated that ferroptosis acted as a “demise change” on this focused PDT (Scheme 1).
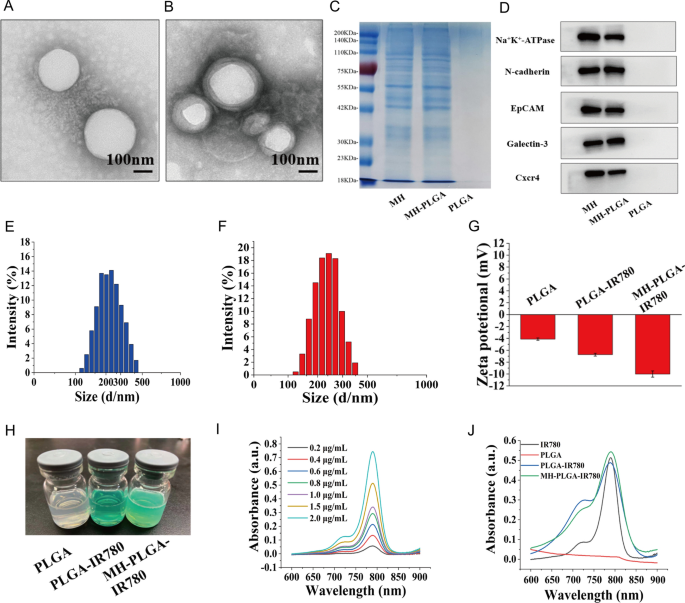
The morphology of the obtained NPs was noticed by TEM (Fig. 1A, B). We discovered that the entire NPs had been uniform in measurement and monodispersed, and that there was a lipid shell on the skin of the NPs wrapped by the cell membrane. Then, we analyzed the general protein elements within the PLGA NPs, MH, and MH-PLGA NPs by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). As proven in Fig. 1C, in contrast with the dearth of protein expression in NPs with out cell membrane coating, the expression ranges of proteins within the MH and MH-PLGA NPs teams had been comparable, which additional confirmed profitable encapsulation within the HOS cell membrane. To confirm the presence of particular homologous-binding adhesion molecules on MH-PLGA-IR780 NPs, we examined the expression of cell membrane markers and a few mobile adhesion molecules (Na+/Ok+-ATPase, Ncadherin, Galectin-3, EpCAM, and Cxcr4) for source-cell-specific concentrating on by way of the homologous binding mechanism by western blot evaluation. As proven in Fig. 1D, the aforementioned proteins within the MH-PLGA-IR780 NPs group had comparable expression to the cell membrane vesicle group, whereas NPs with out cell membrane coating didn’t possess a protein profile, which demonstrated the soundness and effectiveness of cell membrane proteins on MH-PLGA-IR780 NPs by extrusion coating. The particle sizes and zeta potentials of those NPs had been measured by DLS, and the typical sizes of PLGA-IR780 NPs and MH-PLGA-IR780 NPs had been distributed at roughly 218.2 nm and 236.8 nm, respectively (Fig. 1E–F), which is according to the scale decided by TEM. DLS additionally confirmed that the typical sizes of the PLGA NPs had been narrowly distributed and centered at 202.1 nm (Further file 1: Fig. S1A), suggesting that the ensuing common sizes of those NPs would enable them to go by way of the tumor endothelial area [26]. Subsequent, to look at the soundness of the synthesized NPs, they had been positioned in both DMEM (10% serum) or PBS at 4 °C for one week. Their imply measurement measured day by day didn’t change considerably throughout the 7-day evaluation interval (Further file 1: Fig. S1B–C), suggesting that the obtained NPs had good stability underneath physiological situations. As well as, the zeta potentials of the PLGA NPs and PLGA-IR780 NPs had been − 4.62 ± 0.79 mV and − 6.70 ± 0.50 mV, respectively, whereas that of the MH-PLGA-IR780 NPs decreased to − 10.09 ± 0.70 mV (Fig. 1G), which was probably brought on by the detrimental cost from the cell membrane and the minimization of the electrostatic repulsion of IR780. The detrimental zeta potential of the MH-PLGA-IR780 NPs is useful for correct tumor concentrating on as a result of a lower in speedy elimination by the reticuloendothelial system (RES) and prolongation of the time within the systemic circulation [27]. The colour of PLGA NPs and NPs-coated IR780 modified from white to inexperienced (Fig. 1H). The UV–vis spectrum confirmed that the PLGA-IR780 NPs and MH-PLGA-IR780 NPs had a attribute absorption peak at 798 nm from IR780, additional indicating the profitable loading of IR780, not like the PLGA NPs, which didn’t present this attribute absorption peak (Fig. 1J). The absorbance of IR780 modified in a concentration-dependent method, as decided by UV–vis spectroscopy, and a calibration curve was constructed (Figs. 1I, Further file 1: S1D). Lastly, the EE and LC of IR780 had been calculated to be 67.8% and three.25 wt%, respectively.
Characterizations of the MH-PLGA-IR780 NPs. A, B TEM pictures of PLGA NPs and MH-PLGA NPs. (scale bar: 100 nm). C SDS-PAGE protein evaluation outcomes. D Western blot evaluation of membrane-specific protein markers. E, F Dimension distributions of the PLGA-IR780 NPs and MH-PLGA-IR780 NPs had been measured by DLS. G, H Zeta potential (n = 3) and digital pictures of various NPs (PLGA NPs, PLGA-IR780 NPs, and MH-PLGA-IR780 NPs). I Absorbance spectra of IR780 in a concentration-dependent method as recorded by UV–vis spectroscopy. J UV–vis spectra of single IR780, PLGA NPs, PLGA-IR780 NPs, and MH-PLGA-IR780 NPs
Biosafety evaluation of the MH-PLGA-IR780 NPs
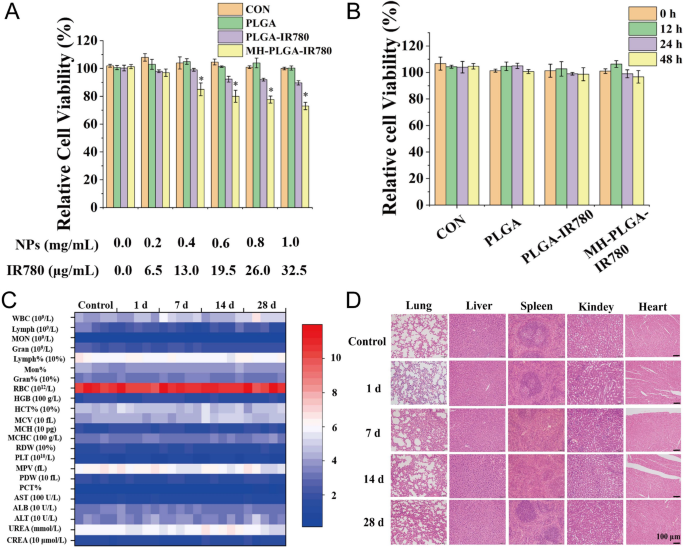
To confirm the protection of the MH-PLGA-IR780 NPs in vitro, a CCK-8 assay was used to look at cell viability 12 h after coincubation of a variety of concentrations of various NPs (PLGA: 0.0, 0.2, 0.4, 0.6, 0.8, 1.0 mg/mL) with HOS cells. As proven in Fig. 2A, the PLGA NPs had no toxicity in HOS cells, whereas IR780-encapsulating NPs exhibited weak cytotoxicity at a better focus (PLGA-IR780 NPs ≥ 0.6 mg/mL) due to the potential cytotoxicity of IR780, which was extra apparent within the MH-PLGA-IR780 NP (NPs ≥ 0.4 mg/mL) therapy [28]. Then, a neutralized focus of the NPs (PLGA: 0.2 mg/mL) was used to find out the cell viability at totally different therapy occasions (0, 12, 24, 48 h). The CCK-8 outcomes demonstrated that the cell viability didn’t change with rising coincubation time. Moreover, there are numerous ligands and receptors on the floor of the most cancers cell membranes which may trigger biosafety dangers, so MH-PLGA-IR780 NPs (PLGA: 5 mg/mL, 200 μL) had been intravenously injected into BALB/c nude mice, and blood samples and main organs (coronary heart, liver, spleen, lung and kidney) had been harvested at 0, 1, 7, 14, and 28 days put up injection. The routine blood examination and serum biochemical index outcomes confirmed no important variations between the samples, and no apparent histopathological adjustments had been noticed within the aforementioned very important organs after therapy with the MH-PLGA-IR780 NPs, indicating acceptable biocompatibility and biosafety in vivo (Fig. 2C, D).
Biosafety of the MH-PLGA-IR780 NPs. A Relative viability (%) of HOS cells after coincubation with a variety of NPs concentrations. B Relative viability (%) of HOS cells after coincubation with MH-PLGA-IR780 NPs (PLGA: 0.2 mg/mL) at extended time factors. (The information are introduced because the imply ± SD). C, D Blood indexes (routine blood and biochemistry) and H&E staining of the primary organs (coronary heart, liver, spleen, lung and kidney) of BALB/c nude mice had been collected at 0, 1, 7, 14, and 28 days after put up injection of MH-PLGA-IR780 NPs. (n = 5). The dimensions bars signify 100 µm
Homologous concentrating on improves intracellular uptake of the MH-PLGA-IR780 NPs
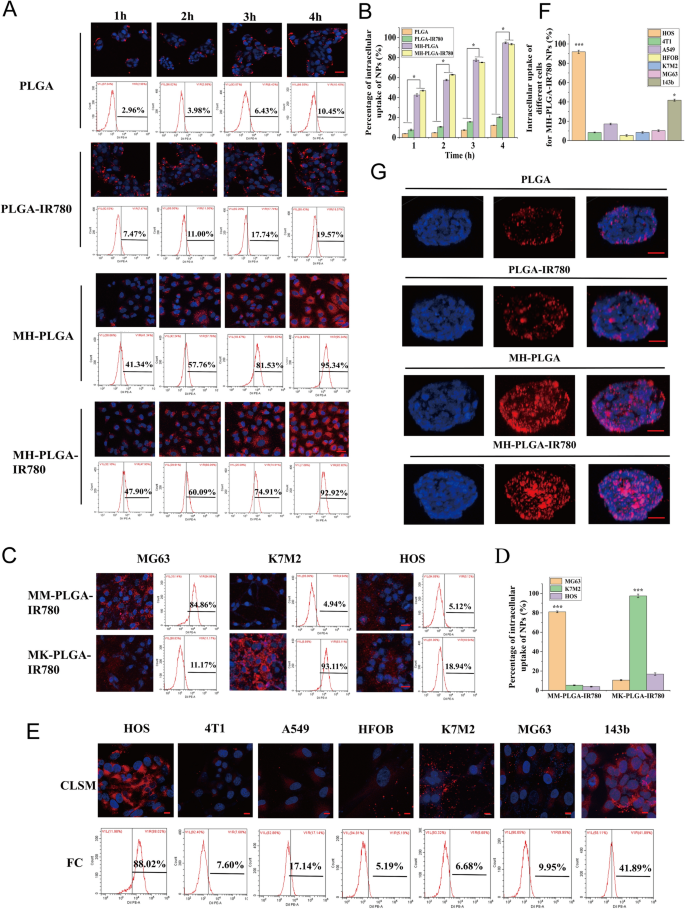
The essence of homologous concentrating on is ligand-receptor interactions primarily based on the overexpressed floor antigens of most cancers cell membranes. After binding to the goal, the constructed NPs can be internalized into the cell by way of ligand-receptor-mediated endocytosis [15, 29]. In abstract, homologous concentrating on narrows the time of intracellular endocytosis and contributes to efficient phagocytosis. Subsequently, to find out whether or not the MH-PLGA-IR780 NPs possessed excessive affinity for the HOS cell line, the mobile uptake effectivity of the MH-PLGA-IR780 NPs (labelled with DiI) was explored by CLSM and FC. As proven in Fig. 3A, B, in contrast with no apparent change in purple FL depth in PLGA NPs and PLGA-IR780 NPs, the purple FL depth within the cells coincubated with MH-PLGA NPs and MH-PLGA-IR780 NPs elevated considerably with extended incubation time, with an uptake fee of larger than 90% after 4 h of coincubation, as decided by quantitative evaluation by FC, indicating that encapsulating the NPs with the HOS cell membrane might resolve the issue of the poor affinity of the normal NPs for the HOS cell line.
A, B Intracellular uptake of PLGA NPs, PLGA-IR780 NPs, MH-PLGA NPs and MH-PLGA-IR780 NPs (labelled with DiI) within the HOS cell line as measured by CLSM and FC evaluation at extended coincubation occasions. The dimensions bars are 50 µm. Statistical analyses of the intracellular uptake fee of the 4 NPs. (The information are introduced because the imply ± SD worth, n = 3, *p < 0.05). C, D Homologous concentrating on capability of PLGA-IR780 NPs wrapped by different OS (MG63, K7M2) cell membranes. The dimensions bars signify 50 µm. Statistical analyses of the intracellular uptake fee of the MK-PLGA-IR780 and MM-PLGA-IR780 NPs (labelled with DiI) to MG63, K7M2 and HOS cell traces. (The information are introduced because the imply ± SD values, n = 3, *p < 0.05). E, F The homologous concentrating on capability of MH-PLGA-IR780 NPs (labelled with DiI) was verified by coincubation with HOS, 4T1, A549, HFOB, K7M2, MG63 and 143b cell traces for 4 h. The dimensions bars signify 10 µm. Statistical analyses of the intracellular uptake fee of MH-PLGA-IR780 NPs to HOS, 4T1, A549, HFOB, K7M2, MG63 and 143b cell traces. G The penetration functionality of PLGA NPs, PLGA-IR780 NPs, MH-PLGA NPs and MH-PLGA-IR780 NPs in HOS 3D tumor spheroids was noticed by CLSM. The dimensions bars signify 50 µm
On account of tumor heterogeneity, the expression ranges of floor antigens within the cell membranes liable for multicellular aggregation formation in tumors are comparatively diversified. The most cancers cell membrane ought to solely have homologous concentrating on capacity to the identical cell line to verify that homologous concentrating on certainly happens in an OS cell traces. On the one hand, the OS cell traces MG63 and K7M2 had been used to organize the corresponding OS cell membranes coated with PLGA-IR780 NPs (MM-PLGA-IR780 NPs, MK-PLGA-IR780 NPs) to look at homologous concentrating on capacity. Three OS cell traces (MG63, K7M2, and HOS) had been individually coincubated with MM-PLGA-IR780 NPs and MK-PLGA-IR780 NPs (labelled with DiI), imaged utilizing CLSM, and quantitatively analyzed by FC. As proven in Fig. 3C, D, CLSM confirmed that MM-PLGA-IR780 NPs solely exhibited a robust purple FL depth within the MG63 cell line, whereas HOS and K7M2 cell traces confirmed weak FL intensities after coincubation with MM-PLGA-IR780 NPs. FC evaluation additional demonstrated that the intracellular uptake fee of the MG63 cell line reached 84.86%, whereas that of the K7M2 and MG63 cell traces was 4.94% and 5.12% after coincubation with MM-PLGA-IR780 NPs. As well as, solely K7M2 introduced an apparent purple FL depth after coincubation with MK-PLGA-IR780 NPs, the intracellular uptake fee of which reached 93.11%. Nevertheless, the opposite two OS (MG63, HOS) cell traces confirmed weak purple FL intensities, and the intracellular uptake charges had been 11.17% and 18.94%, respectively. These outcomes revealed the nice binding capacity of MM-PLGA-IR780 NPs and MK-PLGA-IR780 NPs to the unique supply of OS cell traces.
However, MH-PLGA-IR780 NPs ought to solely exhibit a homologous concentrating on capacity to the HOS cell line owing to their functionalization by adhesion proteins from the HOS cell membrane. To confirm this speculation, PLGA-IR780 NPs with HOS cell membrane coating had been coincubated with totally different tumor cell traces (human non-small-cell lung most cancers A549 and murine breast most cancers 4T1cells), different OS cell traces (MG63, 143b, K7M2) and human osteoblasts (HFOB 1.19). As proven in Fig. 3E, CLSM pictures confirmed that the purple FL depth (MH-PLGA-IR780 NPs) rose and gathered dramatically after 4 h of coincubation with the HOS cell line. Quantitative evaluation confirmed an intracellular uptake fee by FC of 88.02%, whereas unsatisfactory purple FL intensities had been noticed within the 4T1 and A549 cell traces, with intracellular uptake charges of seven.60% and 17.14%, respectively. Furthermore, solely a suboptimal improve (41.89%) was discovered within the purple FL depth within the 143b cell line, probably as a result of the 143b cell line is an HOS cell line with k-ras oncogenic transformation [30]. Poor intracellular uptake was noticed in MG63 (9.95%), K7M2 (6.68%), and HFOB 1.19 (5.19%) cells (Fig. 3E, F), indicating that the PLGA-IR780 NPs wrapped by HOS cell membranes solely have the potential capacity to realize tumor concentrating on within the HOS cell line. Collectively, these outcomes confirmed that the homologous concentrating on capacity of OS cell membrane-coated NPs relies on the supply of the OS cell traces.
Furthermore, hypertension at tumor websites brought on by tumor vascular heterogeneity and a rise within the interstitial strain additional restrict the penetration depth and width of NPs within the tumor area [31]. It has been reported that NPs with homologous concentrating on capability can obtain surface-to-core penetration of tumor cells [16, 32]. Thus, the next experiments had been carried out to guage whether or not the most cancers cell membrane coating was fascinating to enhance the deep penetration capacity of the PLGA-IR780 NPs, which was carried out utilizing 3D tumor sphere fashions to simulate the advanced situations of tumor websites. We cultured HOS 3D tumor spheroids in vitro to look at the penetration functionality of the totally different NPs. As proven in Fig. 3G, CLSM observations demonstrated that PLGA NPs and PLGA-IR780 NPs (labelled with DiI) didn’t exhibit deep penetration functionality, whereas the NPs with a cell membrane coating penetrated into the middle of the HOS 3D tumor spheres for uniform distribution all through the tumor cells, suggesting that the superior penetration depth and width of NPs in HOS 3D tumor spheroids will be achieved by homologous concentrating on. Moreover, the immune escape functionality of various NPs was evaluated in RAW 264.7 murine macrophage-like cells. RAW 264.7 cells had been incubated with numerous NPs for 4 h after which imaged with CLSM. The purple FL depth within the PLGA NPs group was brighter than that within the PLGA-IR780 NPs group, whereas the MH-PLGA-IR780 NPs might keep away from phagocytosis by RAW 264.7 cells and confirmed the least intense purple FL depth (Further file 1: Fig. S2), indicating that coating NPs with the most cancers cell membrane can endow them with the antiphagocytic functionality of RAW 264.7 cells, probably as a result of discount in immune clearance. In conclusion, the MH-PLGA-IR780 NPs achieved tumor concentrating on of HOS cells by way of homologous concentrating on by advantage of ligand receptors on the cell membrane.
In vivo biodistribution by FL imaging
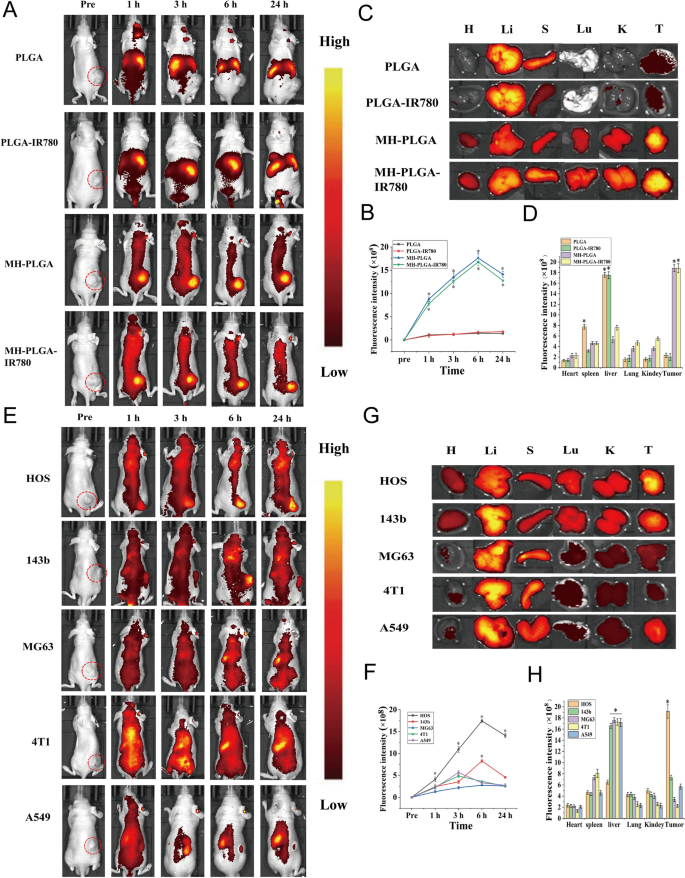
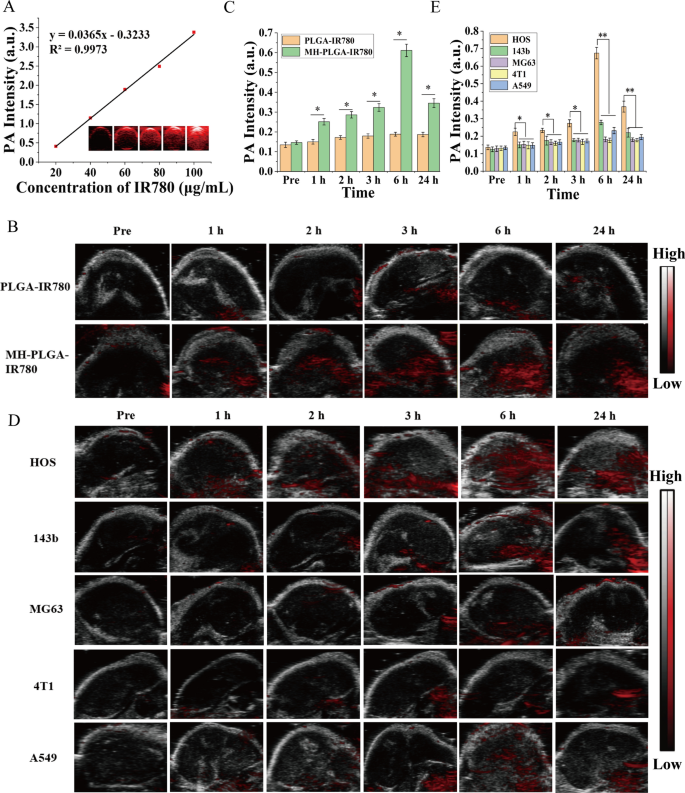
IR780 has been used as a fluorescent probe to impart NPs with NIR FL (λ excitation/λ emission = 745 nm/820 nm) imaging capabilities to guage their biodistribution in vivo, which might additional obtain real-time monitoring of their dynamic distribution to make sure passable supply for therapeutic efficiency [3]. Therefore, totally different NPs had been intravenously injected into HOS tumor-bearing mice to detect the biodistribution of the NPs in vivo after extended intervals of time and to confirm the optimum time for NPs to build up in tumors for additional PDT. As proven in Fig. 4A, B, NPs with out cell membrane coating primarily amassed within the liver and spleen 24 h put up injection, the 2 major organs of the phagocyte-enriched reticuloendothelial system (RES) [33]. In contrast with PLGA NPs and PLGA-IR780 NPs, the strongest FL alerts started to combination throughout the tumor areas as a result of amassed NPs (MH-PLGA NPs and MH-PLGA-IR780 NPs) in a time-dependent method and peaked at 6 h put up injection. Then, the FL depth at tumor websites regularly weakened. Furthermore, the very important organs and tumors of mice had been harvested 6 h post-injection for ex vivo FL imaging to additional affirm the biodistribution of various NPs, and it was discovered that the FL intensities of NPs wrapped by cell membrane in tumors had been larger than these of the guts, liver, spleen, lung and kidney (Fig. 4C, D), which could possibly be attributed to the “homing affinity” of most cancers cell membrane to tumor websites in vivo. Nevertheless, the FL intensities of NPs with out cell membrane coating primarily amassed within the liver and spleen, exhibiting the identical phenomenon because the FL alerts in vivo and suggesting the superior concentrating on properties of NPs with cell membrane coating in vivo.
FL imaging of the totally different NPs in vivo. A, B FL pictures of HOS tumor-bearing mice and quantitative FL sign intensities inside tumor areas earlier than and after intravenous injection of various NPs at extended time factors. C Ex vivo FL imaging of the tumor and main organs harvested from mice at 6 h put up injection. D Semiquantitative biodistribution of tumors and main organs ex vivo was measured by the typical FL intensities. (The information are introduced because the imply ± SD values, n = 3, *p < 0.05). Biodistribution of MH-PLGA-IR780 NPs after intravenous injection in numerous tumor-bearing mice. E, F FL pictures and quantitative FL sign intensities of tumor areas at corresponding time factors in vivo. (The information are introduced because the imply ± SD values, n = 3, *p < 0.05). G, H Ex vivo FL pictures and semiquantitative evaluation of main organs and tumors harvested from mice 6 h post-injection of MH-PLGA-IR780 NPs. (The information are introduced because the imply ± SD values, n = 3, *p < 0.05)
It has additionally been reported that homologous tumor-targeting capacity in vivo reaches a peak at 24 h in human breast most cancers MCF-7 cells [32, 34]. Basically, the differential expression of most cancers cell membrane proteins possesses separate intercellular homologous binding capabilities, which could possibly be utilized for NPs floor functionalization to supply the benefit of full replication of floor antigenic range. Nevertheless, the particular homologous binding mechanism in OS was unclear and is the primary focus of our subsequent research.
In vivo biodistribution by PA imaging
In organic tissues, PA imaging has superior distinction, decision and penetration, enabling the mixing of prognosis and therapy by figuring out the exact location of the medication within the focused area of the tumor [35]. Subsequently, PA imaging was recorded to additional make clear the distribution of the MH-PLGA-IR780 NPs in vivo. Based on associated analysis, the optimum excitation wavelength of NPs coating IR780 for PA imaging was roughly 800 nm. Then, the PA sign of NPs was measured clearly in a dose-dependent method with a superb linear relationship (Fig. 5A), rising from 0.410 to three.382 with rising NPs focus (5, 10, 20, 40, and 80 μg/mL). For PA imaging in vivo, an apparent PA sign depth started to concentrate on the tumor area as a result of amassed MH-PLGA-IR780 NPs at 1 h put up injection and peaked at 6 h put up injection, however solely a weak PA sign in tumor tissue was noticed within the mice handled with PLGA-IR780 NPs (Fig. 5B, C), according to the FL alerts in vivo.
A PA pictures and the corresponding sign intensities of MH-PLGA-IR780 NPs at numerous concentrations. B, C PA pictures and quantitative PA sign intensities of tumor areas earlier than and after intravenous administration of PLGA-IR780 NPs and MH-PLGA-IR780 NPs at corresponding time factors. (The information are introduced because the imply ± SD values, n = 3, *p < 0.05). D, E PA picture and quantitative PA sign intensities of various tumor-bearing mice earlier than and after the injection of PLGA-IR780 NPs and MH-PLGA-IR780 NPs. (The information are introduced because the imply ± SD values, n = 3, *p < 0.05, **p < 0.01)
To additional exhibit the homologous concentrating on capacity of MH-PLGA-IR780 NPs to xenograft tumors in vivo, MH-PLGA-IR780 NPs had been intravenously injected individually into HOS, 143b, MG63, 4T1, and A549 tumor-bearing nude mice, and FL and PA pictures had been then acquired and quantitatively analyzed. As proven in Figs. 4E, F and 5D, E, apparent FL and PA alerts had been noticed within the tumor areas of HOS xenograft tumors even at 1 h put up injection and reached a most at 6 h put up injection. Nevertheless, MG63, 4T1 and A549 tumor-bearing mice exhibited weak FL and PA intensities in tumor areas at every put up injection time level. Owing to the k-ras oncogenic transformation, the FL and PA intensities within the tumor areas of 143b xenograft tumors had been larger than these of MG63, 4T1, and A549 xenograft tumors, which additionally peaked at 6 h put up injection. To additional quantitatively analyze the biodistribution of MH-PLGA-IR780 NPs in numerous xenograft tumors, tumors and main organs of the sacrificed mice had been harvested for ex vivo FL imaging 6 h put up injection. The semiquantitative outcomes confirmed that MH-PLGA-IR780 NPs primarily amassed on the tumor websites of HOS xenograft tumors at ranges roughly 2.8-, 5.1-, 5.7-, and 4.1-fold larger than these within the 143b, MG63, 4T1, and A549 xenograft tumors, respectively. The quantity of MH-PLGA-IR780 NPs of the aforementioned xenograft tumors primarily amassed within the liver, which was larger than these of HOS xenograft tumors, rising by 2.6-, 2.8-, 2.8-, and a pair of.8-fold, respectively (Fig. 4G, H). In conclusion, the outcomes of each FL and PA imaging verified that the MH-PLGA-IR780 NPs would possibly successfully accumulate within the tumor area in vivo by homologous concentrating on, laying a basis for additional tumor diagnostic imaging and focused PDT. Though the present work solely used NPs with a HOS cell membrane coating to confirm the homologous concentrating on capacity in vivo, it’s envisioned that the homologous concentrating on capacity of different OS cell membrane coatings could possibly be efficient.
Homologous concentrating on enhanced IR780-mediated PDT efficiency by mitochondrial dysfunction
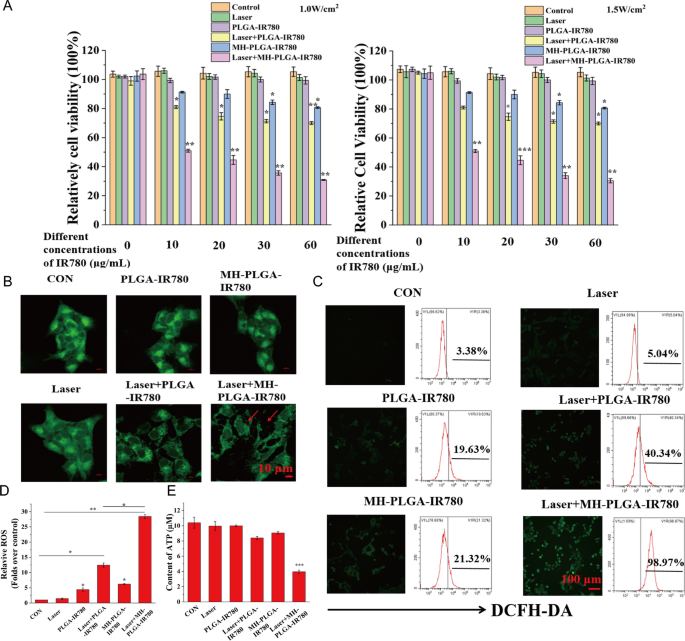
After tumor concentrating on was achieved by homologous concentrating on, a CCK-8 assay was used to estimate the antitumor efficacy of PDT in vitro. As proven in Fig. 6A, in comparison with no apparent change within the management, laser alone, and single NPs with out NIR irradiation teams, the MH-PLGA-IR780 NPs uncovered to laser (focused PDT group) decreased the viability of HOS cells in an IR780 dose-dependent method at energy densities of 1 W/cm2 and 1.5 W/cm2, whereas laser + PLGA-IR780 NPs (PDT with out concentrating on group) confirmed weak photoinduced cytotoxicity in HOS cells. Contemplating that using single MH-PLGA-IR780 NPs alone at a focus larger than 10 μg/mL had weak cytotoxic results on HOS cells after 12 h of coincubation, MH-PLGA-IR780 NPs (5 μg/mL) had been chosen to look at the efficacy and particular mechanism of MH-PLGA-IR780 NPs-mediated PDT within the following experiments.
PDT efficiency with the help of homologous concentrating on. A Relative cell viability (%) of HOS by numerous remedies. B Aggravated mitochondrial fragmentation of HOS cells after focused PDT. The dimensions bars signify 10 µm. C, D CLSM imaging and FC evaluation of the manufacturing of ROS ranges in HOS cells (stained with DCFH-DA) after totally different remedies. The dimensions bars signify 100 µm. (The information are introduced because the imply ± SD values; n = 3, *p < 0.05, **p < 0.01.) E The ATP content material in HOS cells after totally different remedies (the information are introduced because the imply ± SD values; n = 3, ***p < 0.001)
First, the morphology of the mitochondria was noticed by CLSM. As proven in Fig. 6B, in contrast with the physiological morphology of mitochondria within the different 5 teams, mitochondrial fragmentation was present in HOS cells upon focused PDT, which might additional result in mitochondrial dysfunction. As well as, PDT efficiency was detected by DCFH-DA, a probe that detects intracellular ROS era by exhibiting inexperienced FL. As proven in Fig. 6C, D, in contrast with the dearth of a big alteration within the management and laser alone teams, PDT with out concentrating on confirmed inexperienced FL depth, whereas focused PDT produced robust inexperienced FL depth in HOS cells underneath 808 nm NIR irradiation (1.5 W/cm2), which was accompanied by typical morphological options of apoptotic cells. Furthermore, we quantitatively analyzed the share of intracellular ROS by FC, and in contrast with the parallel teams, the quantity of ROS produced by PDT with out the concentrating on group was 40.34%, whereas this worth after therapy with the focused PDT group was considerably elevated to 98.97%. Owing to the potential cytotoxicity of IR780 even with out laser irradiation at excessive concentrations [28, 36], the 2 single NPs teams exhibited slight ROS accumulation (19.63% and 21.32%). ATP, one of the crucial necessary molecules in power provide, is produced primarily by way of mitochondrial metabolism [37] and performs a key function in tumor proliferation and DNA replication. It has been reported that ATP depletion will increase the sensitivity of tumor cells PDT [38]. To exhibit the adjustments in ATP contents after totally different remedies, a calibration curve of ATP was constructed (Further file 1: Fig. S3A). As proven in Fig. 6E, in contrast with the opposite 5 remedies, solely PDT with homologous concentrating on led to a big lower in intracellular ATP content material. In conclusion, these outcomes urged that homologous targeting-based NPs might enhance the efficiency of PDT by rising intracellular ROS manufacturing and inhibiting ATP synthesis as a result of mitochondrial dysfunction.
MH-PLGA-IR780 NPs-mediated PDT synergistically induced apoptosis and ferroptosis
Cell demise is a basic organic course of that usually together with necrosis, apoptosis, autophagy and ferroptosis. Nevertheless, the particular demise mechanism of this homologous concentrating on NPs-guided PDT is unclear. Synergistic induction of a number of demise modes together with tumor remedy is an efficient technique to enhance PDT efficiency [19]. Subsequently, a CCK-8 assay was carried out to research the primary cell demise modes. Earlier than NIR irradiation, HOS cells had been pretreated with a number of inhibitors of cell demise pathways, together with an apoptosis inhibitor (z-VAD-FMK), a necrosis inhibitor (Nec-1), an autophagy inhibitor (Baf-A1), a ferroptosis inhibitor (Fer-1), and a normal ROS scavenger (NAC), for twenty-four h. As proven in Further file 1: Fig. S3B, the CCK-8 assay confirmed that in contrast with the numerous inhibitory impact of PDT on cell viability, cell viability elevated by totally different quantities after pretreatment with every inhibitor (besides Baf-1) adopted by PDT. Nevertheless, NAC, Fer-1, and z-VAD-FMK considerably protected cells from photodynamic cytotoxicity, whereas Baf-1 promoted the inhibitory results of PDT on cell viability, suggesting that along with apoptosis, ferroptosis could also be one other key cell demise mode mediated by MH-PLGA-IR780 NPs-guided PDT and that autophagy performs a protecting function in PDT.
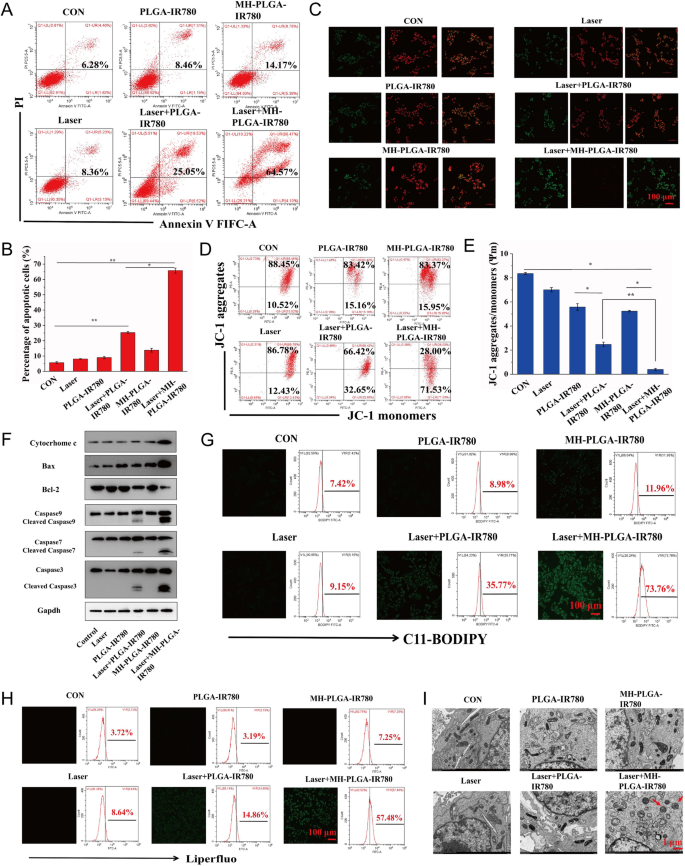
Subsequently, we hypothesized that the constructed homologous concentrating on NP-mediated PDT is fascinating to synergistically induce mitochondrial apoptosis and ferroptosis to kill HOS cells. To additional confirm this assumption and discover the underlying cell demise mechanism, we first measured the HOS cell apoptosis fee by FC utilizing annexin V-FITC/PI double staining. As proven in Fig. 7A, B, the management, NIR irradiation alone, and two NPs with out irradiation teams didn’t exhibit apparent HOS cell apoptosis. The apoptosis fee of HOS cells induced by PDT with out concentrating on was 25.05%, whereas focused PDT elevated the apoptosis fee to 64.57%. Then, contemplating the necessary function Δψm in mitochondrial apoptosis, we measured Δψm with the fluorescent probe JC-1. Throughout mitochondrial apoptosis, mitochondrial membranes are disrupted, with depolarization of Δψm, and mitochondrial depolarization was verified by the lower within the ratio of purple to inexperienced FL depth within the JC-1 assay. As proven in Fig. 7C, E, HOS cells had been stained with JC-1 after totally different remedies. In contrast with the dearth of apparent change within the management, single NIR irradiation, and two NPs alone teams, a slight lower in purple FL depth and a weak improve in inexperienced FL depth had been noticed within the laser + PLGA-IR780 NPs group, and these adjustments had been extra distinguished after therapy with laser + MH-PLGA-IR780 NPs. To additional examine the mechanisms underlying the proapoptotic results of the engineered nanoplatform-mediated PDT, the expression ranges of apoptosis-related proteins had been assessed by western blot evaluation. Laser + MH-PLGA-IR780 NPs therapy considerably elevated the protein ranges of cytochrome c, cleaved caspase-7, cleaved caspase-9, and the proapoptotic protein Bax, accompanied by an apparent downregulation of the antiapoptotic protein Bcl-2, which finally led to a marked improve in caspase-3 cleavage. Nevertheless, the dearth of a distinguished change within the expression ranges of those apoptosis-related proteins within the management, single NIR irradiation, NPs with out irradiation, and PDT with out concentrating on produced unstable pro-apoptotic results (Fig. 7F, Further file 1: Fig. S4).
Analysis of apoptosis and ferroptosis. A, B Induction of apoptosis in HOS cells (stained with annexin V-FITC/PI) after numerous remedies by FC evaluation. (The information are introduced because the imply ± SD values; n = 3, *p < 0.05, **p < 0.01.) C–E Adjustments in Δψm in HOS cells stained with JC-1 after numerous managements, as noticed by way of CLSM and FC. (The information are introduced because the imply ± SD values; n = 3, *p < 0.05, **p < 0.01). The dimensions bars signify 100 µm. F The expression ranges of cell apoptosis-related proteins had been measured by western blot evaluation. G, H The extreme manufacturing of LPO and Lipid ROS in HOS cells after focused PDT as measured by CLSM and FC. The dimensions bars signify 100 µm. I The morphology of mitochondria after numerous remedies as noticed by TEM. The dimensions bars signify 1 µm
Intriguingly, the homologous concentrating on NPs platform developed in our research have the innate benefit of inducing ferroptosis underneath NIR irradiation. On the one hand, the rise in intracellular ROS and the big variety of polyunsaturated fatty acids (PUFAs) within the cell membranes had been discovered to be the important thing drivers of ferroptosis [39], that are inclined to oxidation to kind LPOs. However, mitochondrial dysfunction-mediated ferroptosis has been reported to be an rising technique for most cancers remedy [40]. To additional look at the extent of ferroptosis, LPOs and Lipid-ROS have been acknowledged as essential biomarkers of ferroptosis to impair cell construction and integrity, and each had been detected by liperfluo and C11-BODIPY, respectively [41]. As proven in Fig. 7G, H, CLSM pictures confirmed that the contents of each LPOs and Lipid ROS in HOS cells had been considerably elevated after laser + MH-PLGA-IR780 NPs therapy, as indicated by the numerous improve in inexperienced FL depth, in contrast with an absence of marked alteration within the management, laser alone, and two single NP with out irradiation teams. As well as, weak inexperienced FL was noticed after laser + PLGA-IR780 NPs therapy, which was according to the weak induction of apoptosis by this therapy. Furthermore, quantitative evaluation by way of FC illustrated the identical phenomenon (Further file 1: Fig. S5), indicating that this homologous targeting-based theranostic nanoplatform had superior benefits in inducing ferroptosis underneath NIR irradiation, and the insufficient capacity of PLGA-IR780 NPs mixed with NIR irradiation to induce ferroptosis could also be as a result of lack of mitochondrial dysfunction and PUFAs. Mitochondrial shrinkage is one other necessary attribute of the demise phenotype in ferroptosis [42, 43]. The TEM outcomes additionally demonstrated that the morphology of mitochondria handled with focused PDT grew to become rounded, decreased in measurement, exhibited lowered or absent mitochondrial cristae, and exhibited structural harm, whereas mitochondria within the different 5 teams confirmed a traditional physiological morphology (Fig. 7I). Subsequently, we confirmed that engineered nanoplatform-mediated PDT can considerably and synergistically induce HOS cell apoptosis and ferroptosis.
Focused PDT promoted ferroptosis by inactivating GPX4 and accumulating Fe2+ by ferritinophagy
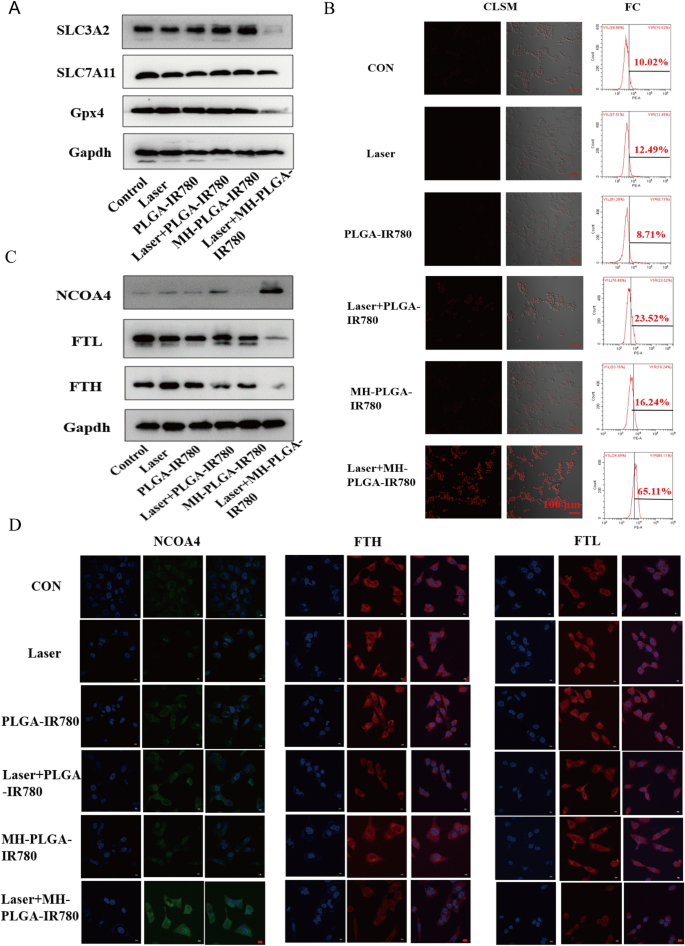
Three of the key elements that have an effect on ferroptosis are tailor-made lipid metabolism, accumulation of redox-active iron (Fe2+), and inactivation of GPX4, a lipid restore enzyme that’s liable for stopping LPO cytotoxicity and sustaining membrane lipid bilayer homeostasis [20]. To additional examine the particular mechanism of ferroptosis induced by MH-PLGA-IR780 NPs-guided PDT, we first detected the protein expression of GPX4 by western blot evaluation, as proven in Fig. 8A and Further file 1: Fig. S6. GPX4 expression was considerably downregulated by laser + MH-PLGA-IR780 NPs therapy, whereas the opposite 5 remedies didn’t obtain equivalent results. SLC7A11 and SLC3A2 are subunits of system XC−, a glutamate/cysteine antiporter that’s liable for sustaining the mobile antioxidant setting, and the low expression of each of those subunits would induce the inactivation of GPX4, stopping LPO manufacturing throughout ferroptosis [44]. Unsurprisingly, in contrast with the parallel therapy teams, PDT with out concentrating on confirmed no affect on both SLC7A11 or SLC3A2 protein expression. Nevertheless, SLC3A2 protein expression was considerably downregulated by laser + MH-PLGA-IR780 NPs therapy, and the protein expression of SLC7A11 was solely barely decreased on this group. Lastly, downregulation of each SLC3A2 and SLC7A11 resulted within the deactivation of GPX4.
The precise mechanism of the induction of ferroptosis by focused PDT. A The expression ranges of ferroptosis-related proteins after totally different remedies detected by western blot. B Enhanced intracellular Fe2+ manufacturing of focused PDT in HOS cells. CLSM pictures (labelled with FerroOrange) and FC evaluation of Fe2+ era after totally different remedies. The dimensions bars are 100 µm. C Western blot evaluation of the expression ranges of ferritinophagy-related proteins (NCOA4, FTH and FTL) after numerous remedies. D IF pictures of NCOA4, FTH and FTL in HOS cells after totally different remedies. The dimensions bars signify 20 µm
As well as, extreme accumulation of redox-active iron in cells induced by mitochondrial dysfunction promotes the manufacturing of extreme ·OH by way of the Fenton response (Fe2+ + H2O2 → Fe3+ + (OH)– + ·OH). ·OH, one other ROS, is ready to oxidize PUFAs, producing LPOs. Thus, we measured the intracellular Fe2+ era functionality of homologous concentrating on nanoplatform-associated PDT by FerroOrange (Japan, colleagues). As proven in Fig. 8B, CLSM evaluation confirmed that in contrast with the parallel teams, the focused PDT considerably elevated the intracellular Fe2+ ranges, as proven by the robust purple FL depth. The quantitative evaluation of FC additionally confirmed that the share of intracellular Fe2+ ensuing from the focused PDT elevated to 65.11%, which was according to the above findings (Further file 1: Fig. S7A). It has been reported that nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy will increase the extent of redox-active iron within the cytoplasm and induces ferroptosis by degrading ferritin [45, 46]. The proteins concerned in iron storage are ferritin heavy chain (FTH) and ferritin gentle chain (FTL). To additional analyze whether or not the particular molecular mechanism of the obtained improve in intracellular Fe2+ degree was related to NCOA4-mediated ferritinophagy, the protein expression ranges of NCOA4, FTH, and FTL had been decided by western blotting. As proven in Fig. 8C and Further file 1: Fig. S7B–D, accompanied by the dearth of apparent adjustments within the management, laser alone, and NPs with out irradiation teams, the PDT with out concentrating on group confirmed a slight improve in NCOA4 expression and a slight discount in FTH expression, whereas the expression of NCOA4 was considerably elevated and the expression of FTH and FTL had been markedly diminished within the laser + MH-PLGA-IR780 NPs group. As well as, the degrees of FTH and FTL expression had been additional confirmed by immunofluorescence (IF) staining. Typically, according to the western blot outcomes, the laser + MH-PLGA-IR780 NPs confirmed important inexperienced FL depth from NCOA4, which was brighter than that within the different 5 teams. Moreover, noticeably weak purple FL intensities of FTH and FTL had been noticed within the laser + MH-PLGA-IR780 NPs group; these ranges had been markedly decrease than these within the management, single NIR irradiation, and two NP with out irradiation teams, whereas the PDT with out concentrating on group confirmed restricted adjustments within the FL intensities of NCOA4, FTH, and FTL (Fig. 8D). In conclusion, we demonstrated that this homologous targeting-associated theranostic nanoplatform not solely can lower the exercise of GPX4 by inhibiting the system Xc− transporter but additionally can promote the buildup of Fe2+ by activating NCOA4-mediated ferritinophagy to degrade ferritin, synergistically inducing ferroptosis in HOS cells.
Antitumor efficacy in vivo
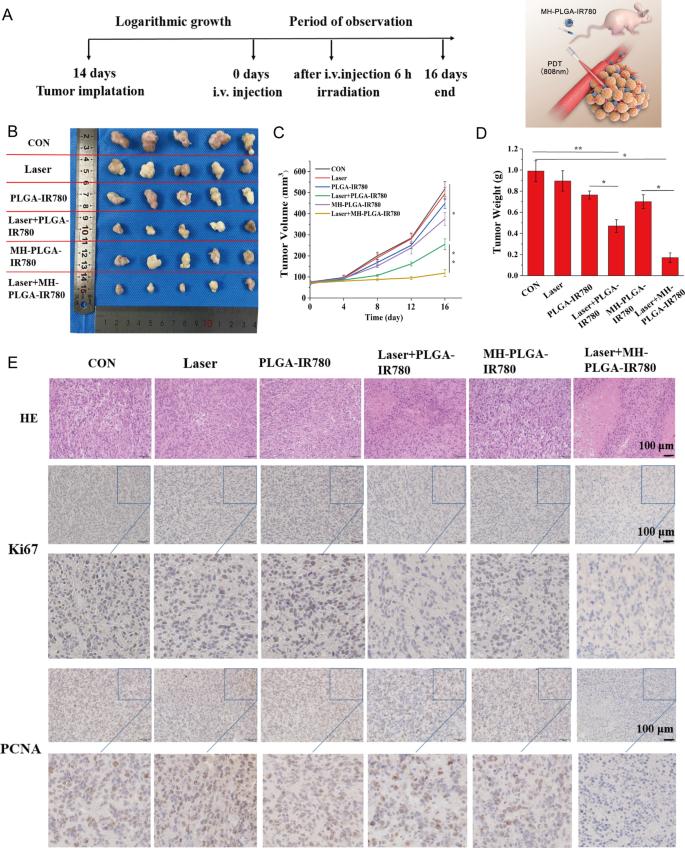
Based mostly on the efficient induction of cell apoptosis and ferroptosis by MH-PLGA-IR780 NPs-mediated PDT in vitro, we examined whether or not an analogous inhibitory impact might happen in a xenograft mannequin. HOS bearing mice had been subcutaneously divided into six teams for various remedies: (1) management (PBS), (2) laser alone, (3) single PLGA-IR780 NPs, (4) laser + PLGA-IR780 NPs, (5) single MH-PLGA-IR780 NPs, and (6) laser + MH-PLGA-IR780 NPs. The tumor areas had been irradiated with an NIR laser (808 nm, 2 W/cm2, 5 min) after 6 h put up injection within the acceptable teams contemplating the PA/FL imaging outcomes. After totally different therapies, the consultant pictures of the mice earlier than sacrifice and tumor visualization had been captured, and tumor volumes had been measured each 4 days over 16 days to observe totally different therapy outcomes (Fig. 9A, B, Further file 1: Fig. S8A). We discovered that tumor development within the PBS and laser alone teams was elevated practically 6.82-fold and 6.13-fold in contrast with the unique tumor volumes, the tumor volumes within the single two NPs teams introduced 5.25-fold and 4.78-fold will increase. Suboptimal tumor development inhibition (certainly, a 3.07-fold improve in tumor quantity) was noticed within the laser + PLGA-IR780 NPs group as a result of lack of concentrating on. In distinction, laser + MH-PLGA-IR780 NPs nearly fully inhibited the expansion of tumors, demonstrating a big tumor inhibition impact in vivo, which is according to the photodynamic cytotoxicity in vitro. Subsequently, the tendencies in tumor measurement and weight had been measured ex vivo and are proven in Fig. 9C, D. No apparent change in mouse weights between the management and handled teams was noticed in the course of the experimental interval (Further file 1: Fig. S8B).
Antitumor capability in vivo. A Schedule of the focused PDT. B Images of tumors harvested from mice after numerous remedies (n = 5) and HOS tumor-bearing mice earlier than sacrifice. C Tumor quantity was measured in the course of the therapeutic interval. (The information are introduced because the imply ± SD values; n = 5, *p < 0.05, **p < 0.01.) D Tumor weights had been recorded on the finish of remedy. (The information are introduced because the imply ± SD values; n = 5, *p < 0.05, **p < 0.01). E H&E staining and pictures of PCNA and Ki67 immunohistochemical staining in tumor specimens after numerous remedies. The dimensions bars signify 100 µm
Moreover, we evaluated the expression of proliferation markers, together with PCNA and Ki67, to additional validate the inhibitory results of various remedies on tumor development. The expression ranges of PCNA and Ki67 in tumor specimens measured by IHC had been decrease within the laser + PLGA-IR780 NPs group than within the management, laser alone, and two single NP teams, whereas the laser + MH-PLGA-IR780 NPs group confirmed the bottom expression. Xenograft tumor samples had been stained with H&E after mice had been sacrificed, as tumor necrosis (karyopyknosis, karyorrhexis, and karyolysis) is an important criterion for evaluating the response to numerous remedies in HOS cells [47]. We discovered that the specimens from the 2 PDT teams confirmed totally different levels of necrosis, whereas specimens from the laser + MH-PLGA-IR780 NPs group exhibited probably the most extreme necrosis (Fig. 9E, Further file 1: Fig. S8C, D). To guage the therapeutic biosafety of PDT in vivo, H&E staining of the primary organs (coronary heart, liver, spleen, lung and kidney) was carried out on the finish of the varied remedies, and routine blood and biochemical evaluation additionally confirmed no important variations among the many numerous teams (Further file 1: Fig. S9). As well as, no obvious histopathological abnormalities had been noticed by way of H&E staining (Further file 1: Fig. S10). Taken collectively, these findings indicated that MH-PLGA-IR780 NPs might improve PDT efficiency in a xenograft mannequin with excessive therapeutic potential and biosafety.
Ferroptosis-promoted focused PDT efficiency
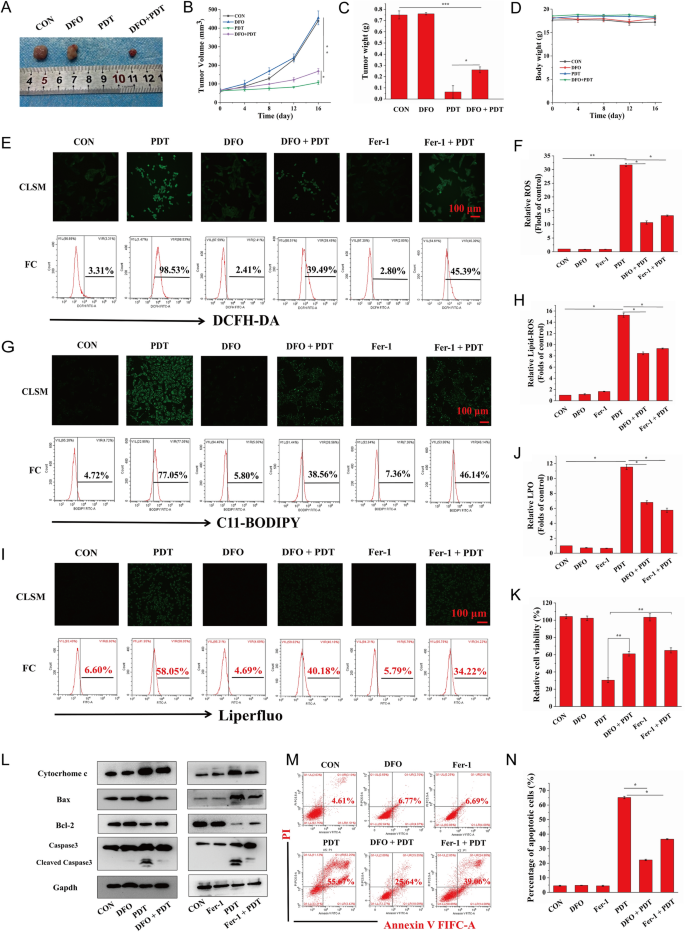
As a brand new mode of RCD, ferroptosis is fascinating to enhance the manufacturing of free radicals by way of the Fenton response, leading to a rise in ROS-mediated oxidative harm to mobile constituents and making certain the excessive effectivity of PDT. To additional decide whether or not ferroptosis induced by focused PDT might improve the unique killing traits of PDT in vivo, we administered PDT-targeted remedy to HOS xenograft tumor-bearing mice after a mix injection of MH-PLGA-IR780 NPs and DFO, a ferroptosis inhibitor that features by chelating iron. The tumor measurement was measured each 4 days over 16 days within the above therapy teams, and it was discovered that in contrast with the numerous inhibitory results of the focused PDT, DFO weakened tumor development within the xenograft mannequin by inhibiting ferroptosis. Visualization and measurement of the weights of the consultant tumors ex vivo confirmed an analogous development, with no important alterations in physique weight (Fig. 10A–D). These outcomes indicated that activation of ferroptosis allowed the engineered nanoplatform-mediated PDT to advertise antitumor results in vivo.
A Consultant pictures of tumors collected from mice after numerous therapies (single DFO dose: 20 mg/kg). B–D Tumor quantity, tumor weight, and mouse weight had been measured in the course of the therapeutic interval. (The information are introduced because the imply ± SD values; n = 5, *p < 0.05, **p < 0.01.) E–J CLSM and FC analyses of the intracellular ROS, LPO, and lipid-ROS ranges by PDT after pretreatments of DFO and Fer-1. (The information are introduced because the imply ± SD values; n = 3, *p < 0.05, **p < 0.01.) The dimensions bars signify 100 µm. Ok The decreased relative cell viability (%) of HOS cells of PDT by way of preincubation of DFO and Fer-1. (The information are introduced because the imply ± SD values; n = 3, **p < 0.01). L After ferroptosis scavenging by DFO and Fer-1, the expression ranges of cell apoptosis-related proteins induced by PDT had been measured by western blot evaluation. M–N The suppression of cell apoptosis percentages (stained with annexin V-FITC/PI) by the inhibition of ferroptosis as decided by FC. The information are introduced because the imply ± SD values; n = 3, *p < 0.05.) PDT: laser + MH-PLGA-IR780 NPs
To research the connection between apoptosis and ferroptosis for PDT in vitro, we sought to make clear whether or not the inhibition of ferroptosis might shield HOS cells from the photodynamic cytotoxic results of the focused PDT. HOS cells had been preincubated with DFO for twenty-four h adopted by focused PDT intervention. Since ferroptosis induction by PDT guided by this nanoplatform was additionally depending on tailor-made lipid peroxidation, HOS cells had been additionally pretreated with one other ferroptosis inhibitor, Fer-1, for twenty-four h earlier than focused PDT intervention. As proven in Fig. 10E–J, each DFO and Fer-1 lowered PDT efficiency by way of intracellular ROS, lipid ROS, and LPOs, as proven by the weaker inexperienced FL intensities in CLSM, according to the outcomes obtained by FC. Contemplating that DFO inhibited ferroptosis by depleting intracellular Fe2+ and not directly blocking lipid peroxidation, the content material of intracellular Fe2+ was detected utilizing FerroOrange, and as proven in Further file 1: Fig. S11, each CLSM imaging and FC analyses revealed the efficient inhibition of intracellular Fe2+ throughout PDT by pretreatment with DFO. In abstract, these outcomes indicated that efficient blockade of ferroptosis would possibly partly shield HOS cells from the cytotoxic results of intracellular ROS, Lipid ROS, and LPOs induced by focused PDT. Moreover, the CCK-8 assay confirmed that pretreatment with Fer-1 or DFO adopted by focused PDT intervention successfully elevated the viability of HOS cells (Fig. 10Ok). Furthermore, to additional examine the connection and the potential signaling pathways between the apoptosis and ferroptosis induced by PDT guided by this nanoplatform, as proven in Figs. 10L and Further file 1: Fig. S12, each Fer-1 and DFO attenuated apoptosis by decreasing cytochrome c and Bax protein ranges, which is according to the upregulation of Bcl-2 protein expression, finally leading to a decreased protein degree of cleaved caspase-3. Lastly, annexin V-FITC/PI double staining evaluation confirmed that HOS cells pretreated with Fer-1 or DFO after which subjected to focused PDT exhibited a marked lower within the whole apoptosis fee in contrast with that of cells subjected to focused PDT with out ferroptosis inhibitor pretreatment (Fig. 10M, N). Taken collectively, these outcomes indicated that the induction of ferroptosis by this theranostic nanoplatform-guided PDT method might contribute to PDT-mediated apoptosis and inhibit tumor development in mouse xenografts.